This Guy ↑↑↑
Why is this so important?
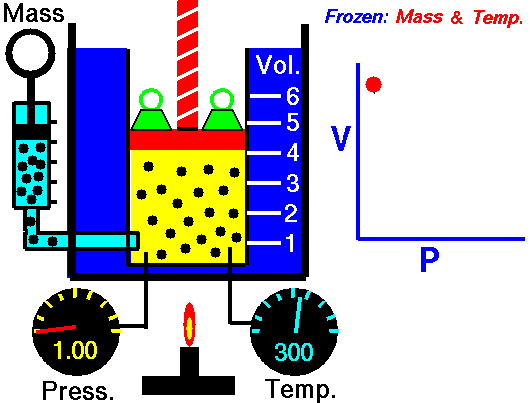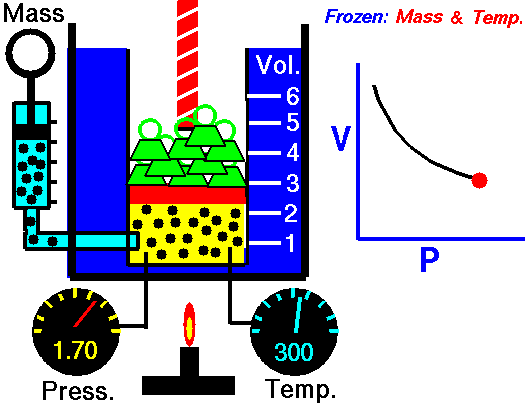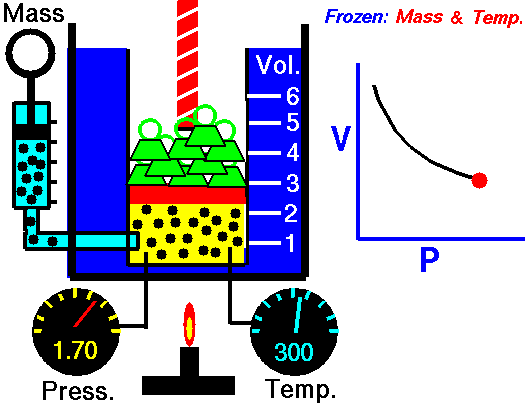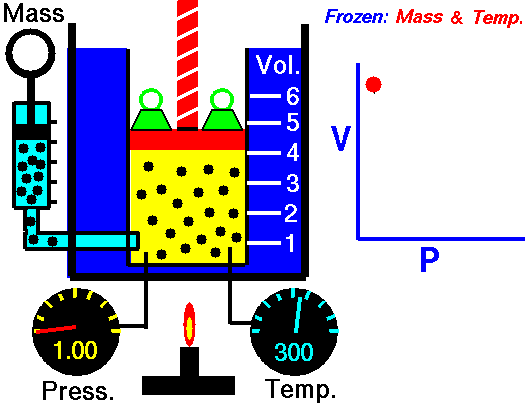
Boyle's Law is one of the few things you learn in school that you actually can see and/or use in real life. You see examples of Boyle's Law when you deep sea dive. When you resurface after deep sea diving the close you get to the surface the bigger the bubbles you exhale are. This is because the pressure of the water on you decreases so the volume of the bubbles increases. That is Boyle's law.
 |
| Boyle's law also explains things like why your ears pop when flying in an airplane and why if you bring a deep sea fish to the surface it dies. |

.gif)







.gif)